
new website for APL and PKMP
A Comprehensive Software Solution for
Pharmacokinetics
Biopharmaceutics
Pharmacodynamics
Clinical Pharmacology
Nonclinical Pharmacokinetics and Metabolism Studies
Bioanalysis and Management
Data Analysis and Reporting
Team Representation and Strategic Planning
Scientific Publications
Regulatory Support ( IND, NDA and MAA)
APL has been incorporated in early 2015 in USA. APL is pharma software solution and consulting services company supporting pharmaceutical research and development. Our software, pharmacokinetic modeling program (PKMP) will support data analysis in the following key areas of drug development: lead identification / optimization, dose response analysis, bioequivalence analysis of products, in vitro-in vivo correlations for formulations, dissolution data analysis, modeling and predictions.
APL has following products and services:
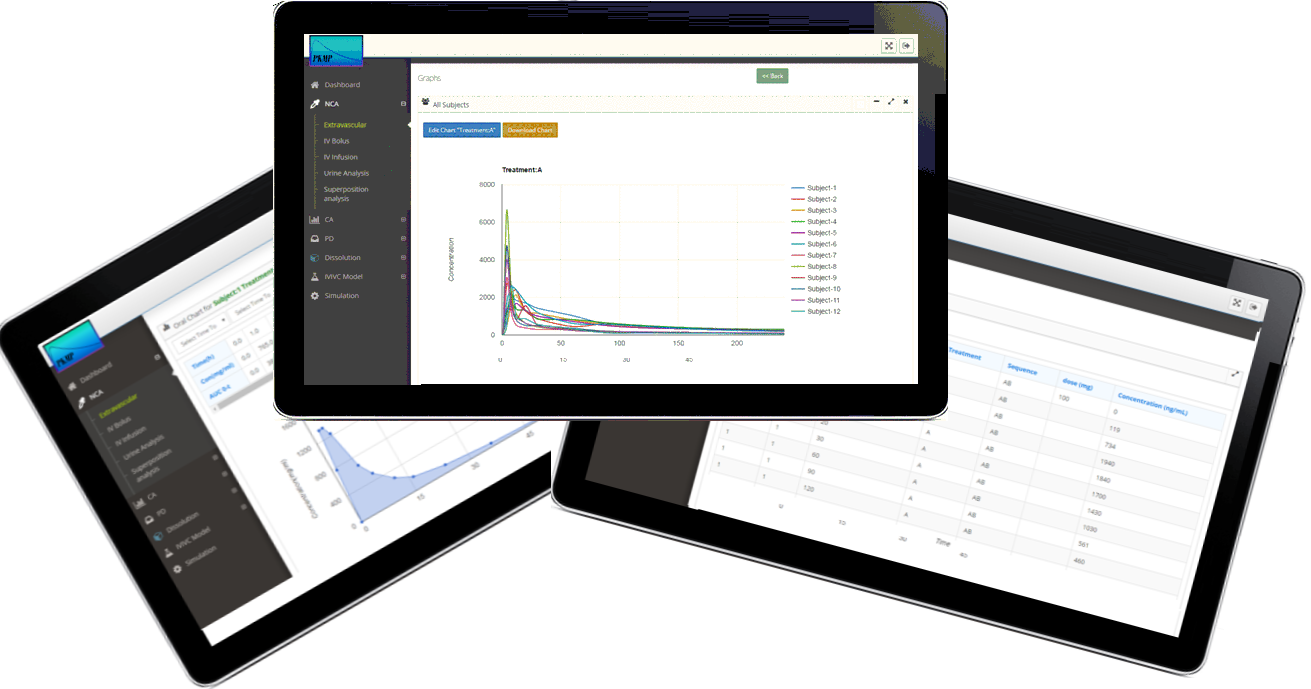
Our software, pharmacokinetic modeling program (PKMP) will support data analysis for pharmacokinetic, clinical pharmacology, biopharmaceutics, and dissolution needs of new drug and generic product developments. These analyses are critical in drug development for cost and time savings.
PKMP will support data analysis in the following key areas of drug development: lead identification/optimization, dose response analysis, bioequivalence analysis of products, in vitro-in vivo correlations for formulations, dissolution data analysis, modeling and predictions.
Our consultancy services include nonclinical and clinical pharmacokinetics, biopharmaceutics, bioanalysis, and drug metabolism aspects during different phases of drug development as well as for generic drug products development. We get involved from planning through execution and regulatory submission needs of pharmaceutical business.
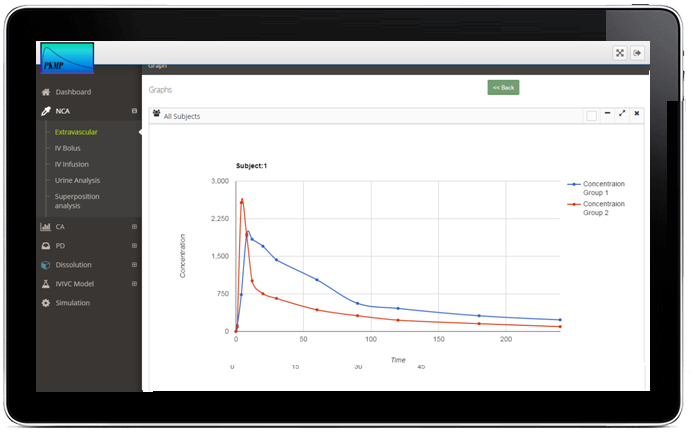
Noncomparmental PK analysis
Compartmental PK Analysis
Dissolution Data Analysis
In vitro – in vivo Correlation
Simulation
Reporting
Validation
Plasma PK Analysis
Urine PK Analysis
Superposition Analysis
Toxicokinetics
Interspecies Scaling
BE 2x2 crossover,
Repeated BE,
Anova,
Dose proportionality
Pharmacokinetics
Oral (1CBM, 2CBM)
Intravenous (1CBM, 2CBM, 3CBM)
Intravenous infusion (1CBM, 2CBM, 3CBM)
Pharmacodynamics
Emax
Sigmoidal Emax
Inhibitory Emax
Sigmoidal inhibitory Emax
Dissolution Models
Profile Comparisons
Multivariate statistical distance
Loo-Riegelman Method
Numeric Deconvolution
Wagner-Nelson Method
Level A correlation
Convolution
Levels A, B, C, and Levy Plots
Pharmacokinetic
Pharmacodynamic
IVIVC
Sample size calculation
Absorption, distribution, metabolism, and elimination
A relationship between the concentration and a response
The process of dissolving a drug substance from the solid state
In vitro –in vivo correlation for modified release dosage forms
Limited sampling data analysis in animal toxicity studies
Evaluating relationships based on mathematical models
A process of checking software system meets specifications
For background and how to perform different analysis
| Sl No. | Name | Version | Release Date | Status | ||
|---|---|---|---|---|---|---|
| 1. | PKMP Release Note | 1.03.58 | 08-Jan-2026 | View | ||
| 2. | PKMP Release Note | 1.03.57 | 07-Nov-2025 | View | ||
| More Releases... | ||||||
Address: APL 9671 Laforet drive, Eden Prairie, MN 55347, USA Email: shah.ajit@aplanalytics.com