new website for APL and PKMP
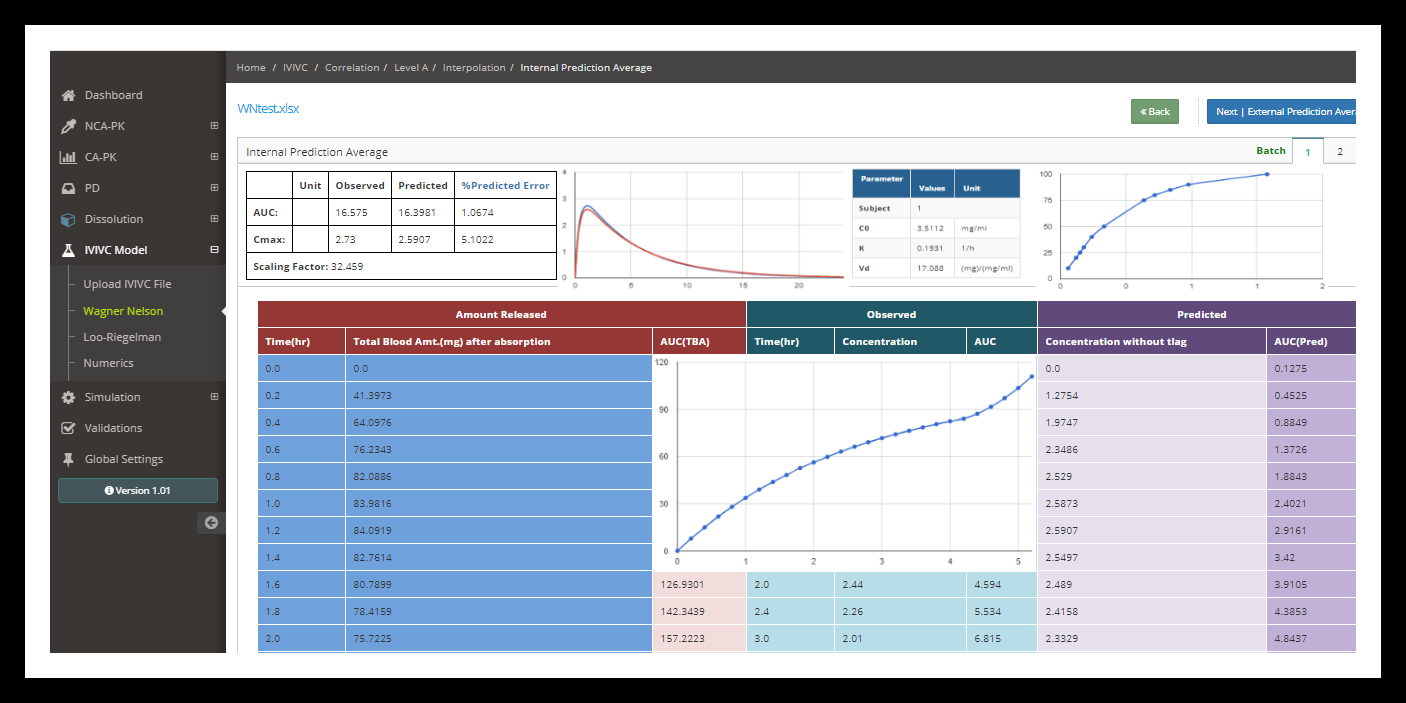
In vitro–in vivo correlation (IVIVC) for modified release dosage forms has been often used during pharmaceutical development in order to reduce development time and optimize the formulation. A good correlation is a tool for predicting in vivo results based on in vitro data.
IVIVC allows dosage form optimization with the fewest possible trials in human, fixes dissolution acceptance criteria and can be used as a surrogate for further bioequivalence studies; it is also recommended by regulatory authorities.