new website for APL and PKMP
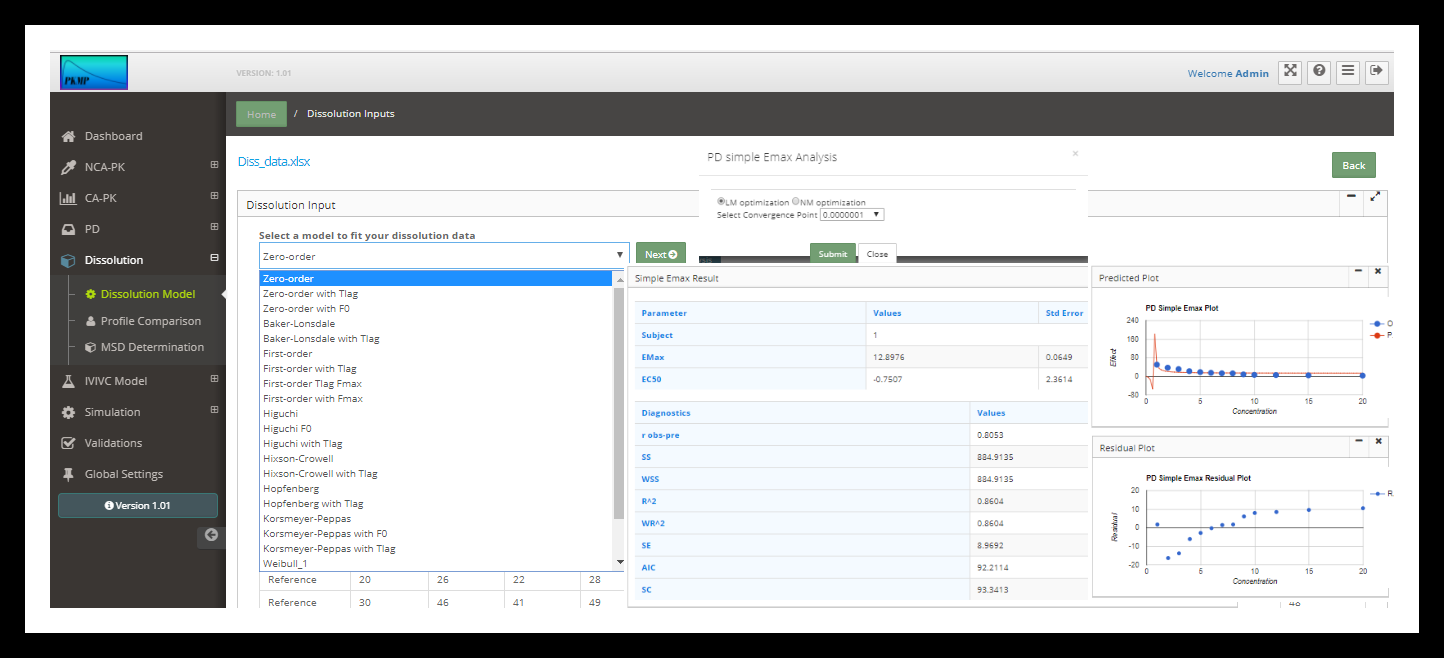
Dissolution is the process of dissolving the drug substance from the solid state. Drug absorption from a solid dosage form after oral administration depends on the release of the drug substance from the drug product, the dissolution of the drug under physiological conditions, and the absorption across the gastrointestinal tract. Because of the critical nature of the first two of these steps, in vitro dissolution may be relevant to the prediction of in vivo performance of drug product. Therefore, in vitro dissolution for immediate release solid oral dosage forms, such as tablets and capsules, are used to